
Back تابون Arabic تابون (عامل اعصاب) AZB Табун (хімічная зброя) Byelorussian Табун (газ) Bulgarian Tabun Czech Tabun German Ταμπούν Greek Tabún Spanish Tabun Basque تابون Persian

| |
| |
| Names | |
|---|---|
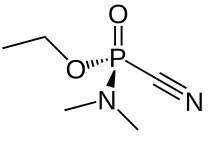
| IUPAC name
(RS)-Ethyl N,N-Dimethylphosphoramidocyanidate
| |
| Other names
GA; Ethyl dimethylphosphoramidocyanidate; Dimethylaminoethoxy-cyanophosphine oxide; Dimethylamidoethoxyphosphoryl cyanide; Ethyl dimethylaminocyanophosphonate; Ethyl ester of dimethylphosphoroamidocyanidic acid; Ethyl phosphorodimethylamidocyanidate; Cyanodimethylaminoethoxyphosphine oxide; Dimethylaminoethodycyanophosphine oxide; EA-1205; TL-1578
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H11N2O2P | |
| Molar mass | 162.129 g·mol−1 |
| Appearance | Colorless to brown liquid |
| Density | 1.0887 g/cm3 at 25 °C 1.102 g/cm3 at 20 °C |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 247.5 °C (477.5 °F; 520.6 K) |
| 9.8 g/100 g at 25 °C 7.2 g/100 g at 20 °C | |
| Vapor pressure | 0.07 mmHg (9 Pa) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly toxic. Fires involving this chemical may result in the formation of hydrogen cyanide |
| NFPA 704 (fire diamond) | |
| Flash point | 78 °C (172 °F; 351 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tabun (military designation GA) is an extremely toxic compound of the organophosphate family.[1][2] It is not present in nature. At room temperature, the pure compound is a clear and viscous liquid. However, impurities imparted during its manufacture are almost always present, turning it into a yellow or brown liquid. Exposed to environs, it slowly volatizes into the atmosphere,[3] with the vapor having a slight fruity or almond-like odor.[4] As the compound has a much higher molecular mass (162 g/mol) compared to air, Tabun gas tends to accumulate in low-lying areas.[4]
It is a potent inhibitor of acetylcholinesterase, a key enzyme within the human body as well as in other animals.[5] Acetylcholinesterase is responsible for breaking down acetylcholine, a neurotransmitter released into the synaptic cleft by motor neurons. The presence of acetylcholine within the cleft signals the post-synaptic (downstream) motor neuron to contract the neuron's associated muscle fibers, and vice versa. By irreversibly phosphorylating the enzyme,[2] Tabun accomplishes a constant and involuntary contraction of the affected muscles, as the acetylcholine is not recycled and continues to build up within the cleft. Death of the organism ensues when respiratory muscles, such as the diaphragm and intercostals, become exhausted and paralyzed from constant contraction, leading to loss of respiratory functions.[2]
The production and storage of Tabun has been strictly regulated under the Chemical Weapons Convention and its implementing agency OPCW since 1997.[6] As a Schedule 1 Toxic Chemical,[7] the synthesis of more than 100 grams of the substance per year must be declared to the organization, and no signing nation can possess more than 1 ton of the chemical.[8] Modern usage of Tabun is limited to research purposes in minute amounts.[9]
- ^ "CBRNE - Nerve Agents, G-series - Tabun, Sarin, Soman: Practice Essentials, Pathophysiology, Epidemiology". Medscape. 2021-10-02.
- ^ a b c "CBRNE - Nerve Agents, G-series - Tabun, Sarin, Soman: Practice Essentials, Pathophysiology, Epidemiology". CBRNE. Pathophysiology. 2021-10-02 – via Medscape.
- ^ "NRT CBRN Tabun 2022 07 26.pdf" (PDF). NRT.org.
- ^ a b "Tabun (GA): Nerve Agent | NIOSH | CDC". www.cdc.gov. 2024-04-26. Retrieved 2024-07-26.
- ^ PubChem. "Tabun". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-07-26.
- ^ "Tabun - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2024-07-26.
- ^ "Schedule 1". OPCW. Retrieved 2024-07-26.
- ^ "Chemical Weapons Convention - CWC_en.pdf". OPCW. p. 4. Retrieved 2024-07-26.
- ^ "Chemical Weapons Convention - CWC_en.pdf". OPCW. p. 124. Retrieved 2024-07-26.
